
DEPARTMENT OF JUSTICE
OFFICE OF THE INSPECTOR GENERAL
INSPECTIONS REPORT
RETENTION OF DRUG EVIDENCE IN DRUG
ENFORCEMENT ADMINISTRATION LABORATORIES
February 1996
Report Number I-96-02
TABLE OF CONTENTS
DEA Drug Evidence Vaults Should Be Adequate to Meet Near Term Drug Evidence Storage Requirements
Many Older Drug Exhibits Are Maintained in DEA Laboratory Vaults.
Some Drug Exhibits Have Been in DEA Vaults Without Activity For Many Years
Exhibits Retained for Completed Cases and Non-Drug Evidence Take Up Space
DEA Should Better Control Drug Evidence Exhibits Signed Out to Court for Extended Periods
Figure 1 - Exhibits and Cases, by Lab
Figure 2 - DEA Drug Evidence Inventory, All Regional Labs
Figure 3 - Exhibits - All Regional Labs, Aging by STRIDE Original Record Date
Figure 4 - Exhibits by Lab, Seized Between 10 and 20 Years Ago
Figure 5 - Exhibits by Lab, Seized More Than 20 Years Ago
Figure 6 - DEA Drug Evidence Signed Out
Table 1 - Changes in Drug Evidence Inventories, by Lab
Table 2 - Vault Storage Capacity and Usage, by Lab
Table 3 - Exhibits Destroyed Over the Past 5 Years
Table 4 - Exhibits With No Activity for More Than 10 Years
Table 5 - Exhibits Signed Out For More Than 2 Years, by Lab
APPENDIX I - Management's Response to Draft Report Report - NOT INCLUDED IN THIS HYPERTEXT VERSION.
APPENDIX II - Office of the Inspector General's Analysis of Management's Response Report - NOT INCLUDED IN THIS HYPERTEXT VERSION.
In response to a request from Drug Enforcement Administration (DEA) management, the Office of the Inspector General, Inspections Division, has completed a review of policies and practices relating to the retention of drug evidence at DEA laboratories. DEA officials expressed concern that the laboratory vaults are becoming crowded and were looking for ways to reduce the amount of old drug evidence stored in these vaults.
We have concluded that DEA has adequate drug evidence storage capacity for the near term. Only one facility, the South Central Laboratory, is likely to experience overcrowding in the near future. The remaining laboratories have experienced declines in the number of exhibits in drug inventories since 1990, or have sufficient unused vault space to accommodate near term growth in the number of drug exhibits.
DEA plans to meet long term storage requirements with a 5-year laboratory replacement program, which includes new, high capacity drug storage vaults. These new vaults should accommodate future drug evidence storage needs at these laboratories. If DEA's plans to replace these laboratories are delayed for budgetary or other reasons, or should the number of exhibits in DEA inventories increase unexpectedly, several of DEA's drug evidence vaults could become overcrowded in the future.
DEA can remove a significant number of the exhibits currently maintained in DEA drug evidence vaults. Almost 8 percent of the exhibits in DEA drug evidence vaults have been stored there for more than 10 years and many of these have not been moved from the vaults during that time. At a minimum, DEA should provide case status information to United States Attorneys' Offices' staff for these 10-year or older cases. Cases that are no longer viable should be closed and the related exhibits should be removed from DEA drug evidence vaults. DEA can remove at least another 7 percent of the exhibits in its drug vaults, including exhibits related to cases that have already been completed and those that do not contain drug evidence.
During the inspection, we found laboratory records showing drug evidence exhibits signed out to DEA enforcement agents for extended periods. According to laboratory records, almost one in four exhibits signed out had been signed out for more than 2 years. Many of these exhibits were apparently transferred to state or local agencies by DEA agents who did not provide notice of the transfer to laboratory personnel responsible for maintaining drug evidence inventories. DEA should take steps to strengthen accountability for exhibits signed out for extended periods and to ensure laboratory records are updated as needed.
DEA also should automate the process for generating drug evidence disposition forms. Relatively minor changes to the automated inventory system to allow for automatic generation of these forms should improve the efficiency of the process. These changes will also reduce the paperwork burden on enforcement operations staff who are currently generating these forms manually.
The Office of the Inspector General (OIG), Inspections Division, has completed an inspection of Drug Enforcement Administration (DEA) policies and practices relating to the retention of drug evidence at DEA laboratories. The DEA requested this inspection based upon the concern that DEA laboratory vaults are becoming crowded and a desire to find ways to reduce the amount of old drug evidence stored in DEA vaults.
In response to DEA's request for this inspection, we designed inspection steps to find out if: (1) vault space is adequate in DEA laboratories, (2) DEA has taken steps to destroy drug evidence for which it has authority under current rules, and (3) opportunities exist for DEA to reduce administrative costs associated with the storage and handling of drug exhibits, especially old drug exhibits.
SCOPE AND METHODOLOGY
On this inspection, we reviewed existing DEA policies and practices concerning the retention of drug evidence. To assess the impact of current policies and practices, we:
-- Interviewed a variety of DEA officials, from both DEA headquarters and field offices, and officials of the Executive Office for United States Attorneys and selected United States Attorneys' Offices;
-- Analyzed data from DEA's automated evidence inventory system and DEA's enforcement case status system;
-- Inspected each DEA drug evidence vault, and examined randomly selected drug evidence exhibits within each vault; and
-- Reviewed case file information for selected exhibits.
Our random sample of drug evidence exhibits included a total of 1,590 exhibits nationwide out of 88,593 exhibits stored as of February 1995 in DEA's seven regional laboratories. We also reviewed case file information for selected exhibits in our sample to determine whether DEA is maintaining drug exhibits for cases previously concluded. Our sample of 1,590 exhibits included 43 exhibits for which the case status code in the automated case status system was listed as "normally closed," 73 for which the case status was listed as "administratively closed," and 32 exhibits for which a "data base record" was created in the automated system to reflect that the exhibit was seized prior to implementation of DEA's automated case status system. [ Cases should be "normally closed" in the system when all investigative and judicial activity has ended and all drug and non-drug evidence has been destroyed or removed from DEA vaults. "Administratively closed" refers to cases which have been closed for some other reason. For instance, cases transferred to task forces are usually closed in the system and reopened under new task force case numbers. ]
We used the number of exhibits in DEA drug evidence vaults as an indicator of the total amount and volume of evidence contained in each vault. Although exhibits do range in size, DEA does not generally store bulk evidence in the drug evidence vaults. Bulk quantities of drugs (other than marijuana) beyond the amounts required for prosecution are routinely destroyed with the approval of the United States Attorneys' Offices. Bulk marijuana is stored outside the laboratories in separate facilities under the control of enforcement offices. The vast majority of exhibits we found in DEA vaults were stored in plastic, heat-sealed envelopes. These envelopes are generally of a uniform size, i.e., 9 inches by 16 inches by 2 inches or less. DEA stores a relatively small number of larger drug exhibits in boxes.
Although we reviewed numerous DEA storage and retention policies, including policies related to the destruction process, we did not review the destruction process itself. We also did not evaluate: (1) DEA policies and practices related to vault security or the chain-of-custody process required for drug evidence in DEA's possession, (2) retention of bulk marijuana evidence that is stored in separate enforcement office warehouses, and (3) the DEA Special Testing Laboratory in McLean, VA. The Special Testing Laboratory provides a variety of specialized scientific, research, and forensic laboratory support services for both foreign and domestic law enforcement agencies, and does not store large amounts of drug evidence.
Because the OIG Audit Division recently issued a report on DEA Laboratory Operations, we relied on the findings of this audit for information on the overall cost of laboratory operations.
As a routine part of criminal investigations, DEA seizes illicit drugs, which are then used as evidence in the prosecution of drug traffickers and users. After seizure, these drugs are sent to DEA laboratories for analysis and are stored in DEA drug evidence vaults for safekeeping.
DEA operates seven regional drug laboratories located in:
· Washington, D.C. (Mid-Atlantic),
· New York, NY (Northeast),
· Miami, FL (Southeast),
· Chicago, IL (North Central),
· Dallas, TX (South Central),
· San Diego, CA (Southwest), and
· San Francisco, CA (Western).
DEA's seven regional drug laboratories serve DEA and other federal agencies, including the Federal Bureau of Investigation and the United States Customs Service. These laboratories also provide drug analysis services to many state and local law enforcement agencies. As of February 1995, DEA's drug vaults in the seven regional laboratories contained 88,593 exhibits related to 15,287 cases (see Figure 1).

DEA stores all drug evidence in secure facilities. When drugs are seized, DEA personnel package the drug evidence into exhibits and DEA chemists analyze each exhibit. After the exhibits are analyzed by DEA chemists, the drugs are retained in the laboratory vaults except during the time they are used in court, or until they are no longer needed as evidence and are removed for destruction. DEA does not destroy drug evidence exhibits unless approval is first obtained from the United States Attorney's Office responsible for the case.
Some non-drug evidence items also are stored in the laboratory vaults. Such evidence includes various items, packages, and containers that may contain trace amounts of illegal drugs, as well as other substances and chemicals that have evidentiary value. DEA stores most non-drug evidence in separate secure storage facilities that are not under the control of the DEA laboratories.
DEA Drug Evidence Storage Costs
The cost of laboratory vault space makes up only a very small part, less than 3 percent, of the total expense DEA incurs to operate its seven regional laboratories and one Special Testing Laboratory. DEA currently pays about $450,000 in annual lease costs for secure, drug evidence vault space. This does not include "in-process"vault space in which exhibits are stored short-term during the time they are analyzed by DEA chemists. In a recent report, the OIG Audit Division listed total DEA laboratory program costs, including space rental, personnel, and all other expenses at $17.4 million in fiscal year 1994, exclusive of headquarters activities.
The OIG Audit Division report concluded that DEA laboratories generally "were housed in aging, outmoded, or obsolete facilities." The Audit Division found "instances of overcrowding; inadequate fume hood space; inadequate ventilation for laboratory personnel, exhibits and chemicals; and insufficient storage space for exhibits, other controlled substances, and chemicals."
Recognizing these conditions at several facilities, DEA expects to replace the Special Testing and Research Laboratory and four regional laboratories: the Mid-Atlantic, Southwest, Southeast, and Western laboratories. The new laboratories, as planned, will include higher capacity drug storage vaults. These new vaults should readily accommodate any increases in DEA drug storage requirements at these laboratories.
Direct funding to replace these five laboratories would cost about $60 million over a 5-year period, according to DEA officials. However, DEA plans to commercially lease new laboratory facilities, built to DEA specifications, and to amortize construction costs over the life of long-term leases. This arrangement should significantly reduce DEA's up-front expenditures and would spread the cost of construction over a period of 20 years or longer.
DEA laboratories maintain a data base, the System to Retrieve Information from Drug Evidence (STRIDE), which they use to track evidence through the laboratory system from receipt through destruction. STRIDE also contains information on evidence at each laboratory, such as: exhibit status, results of analysis, analysis turnaround time, and staff resource expenditures.
DEA laboratories conduct routine annual inventories of the drug evidence stored in the DEA drug evidence vaults. During these annual inventories, laboratory officials check the contents of the vault against a computerized listing of evidence exhibits produced from STRIDE. Discrepancies are subsequently resolved or investigated and appropriate action taken. Similar drug vault inventories are also performed each time a laboratory official, or any individual who has access to the vault, retires or leaves the job.
Also on an annual basis, laboratory officials formally provide DEA enforcement officials with listings of cases for which there is drug evidence in the laboratory vaults. Enforcement agents are then required to assess the status of each of their cases and determine if any cases related to specific drug evidence can be closed. During the annual inventory process, enforcement agents are required to: consult with representatives from the United States Attorneys' Offices, notify the laboratory of the status of each case, and generate a DEA Form 48 (Disposition of Drug Evidence) for exhibits related to concluded cases. DEA regulations provide that cases should not be officially closed until all criminal and civil judicial proceedings have been concluded, and all exhibits related to these cases have been destroyed.
DEA is installing a bar code system, the Laboratory Evidence Management System (LEMS) that will be used to speed up the evidence inventory process. LEMS will create an automated record that is expected to replace the manual Evidence Accountability Record (DEA Form 307) currently maintained for every drug exhibit.
RESULTS OF THE INSPECTION
DEA DRUG EVIDENCE VAULTS SHOULD BE ADEQUATE TO MEET NEAR TERM DRUG EVIDENCE STORAGE REQUIREMENTS
The number of drug evidence exhibits in DEA's regional laboratory vaults has grown less than 3 percent between 1990 and 1994 (see Figure 2).
Given this slow growth in the number of exhibits, existing DEA drug vaults should be adequate in the near term.

Trends in the number of exhibits in DEA drug evidence vaults have varied by regional laboratory. Between the end of calendar year (CY) 1990 and the end of CY 1994, the number of exhibits declined in three of the seven regional laboratories, and increased in the other four.
Table 1 reflects the changes in the number of exhibits stored at each laboratory over this 4-year period.
| Changes in
DEA Drug Evidence Inventories All Regional Laboratories |
|||
| Laboratory | End of Year Exhibits 1990 | End of Year Exhibits 1994 | Percent of Change 1990 through 1994 |
| Mid-Atlantic | 5,127 | 4,381 | -15% |
| Northeast | 22,346 | 20,168 | -10% |
| Southeast | 9,615 | 11,845 | 23% |
| North Central | 12,671 | 13,954 | 10% |
| South Central | 9,797 | 11,418 | 17% |
| Southwest | 15,041 | 12,577 | -16% |
| Western | 11,920 | 13,662 | 15% |
Source: STRIDE - Data Extracted 2/13/95
Total vault space and shelf space for each DEA regional laboratory is listed in Table 2.
| LOCATION | TOTAL VAULT SPACE (SQUARE FEET) |
VAULT SHELF SPACE (SQUARE FEET) |
SHELF SPACE IN USE (PERCENTAGE) |
| Mid-Atlantic | 615 | 1,044 | 90% |
| Northeast | 2,500 | 3,388 | 90% |
| Southeast Off Site Storage |
543 5,000 |
1,074 N/A |
90% N/A |
| North Central | 1,580 | 4,065 | 75% |
| South Central | 2,155 | 3,094 | 95% |
| Southwestern | 1,965 | 3,241 | 95% |
| Western | 1,658 | 3,270 | 66% |
| Totals: | 16,016 | 19,176 | 85% |
Of the laboratories that are filled within 5 or 10 percent of capacity, all but the South Central Laboratory experienced a decline in the number of drug exhibits between 1990 and 1994.2 In the South Central Laboratory, crowded conditions exist despite the additional capacity of several movable shelving units now in use for heat-sealed exhibits. High capacity, moveable shelving units have also been installed in the North Central Laboratory, DEA's newest laboratory. These movable shelving units have helped eliminate overcrowding in the North Central facility. DEA officials told us that using high capacity, movable shelving in other laboratory vaults is not feasible because the added weight of these shelving units would exceed weight bearing limits.
[ 2Although the Southeast laboratory has only 10 percent unused space in its drug vault, the laboratory maintains a large, secure off-site ware house which provides sufficient space to keep up with future drug storage requirements.]
Although this is not an ideal situation, exhibits are sometimes stored using available vault floor space when the inventory of exhibits exceeds available shelf space in the vault. We do not recommend long-term storage of exhibits in this way because cluttered vault aisles can be hazardous and make retrieving and accounting for exhibits more cumbersome and time-consuming.
The planned replacement of four regional laboratories, which will include higher capacity drug storage vaults, should easily meet DEA's future drug storage requirements at these locations. However, the South Central Laboratory drug vault is already filled to near capacity, is not scheduled for replacement, and may experience significant overcrowding in the future. In addition, if DEA's plans to replace these laboratories are delayed for budgetary or other reasons, or should the number of exhibits in DEA inventories increase unexpectedly, several of DEA's drug evidence vaults could become overcrowded. DEA should, therefore, adopt more aggressive space management practices to avoid overcrowding.
DEA SHOULD REMOVE EXHIBITS FROM ITS DRUG EVIDENCE VAULTS
DEA can significantly reduce the number of exhibits contained in its vaults by removing exhibits for old drug cases that are no longer viable, exhibits related to completed cases, and "non-drug" exhibits.
Many Older Drug Exhibits Are Maintained in DEA Laboratory Vaults
As of February 1995, DEA was storing almost 7,000 exhibits, related to about 1,100 cases, which were seized more than 10 years previously (see Figure 3). Our analysis of STRIDE data also identified 576 exhibits, related to 117 cases, that were seized more than 20 years ago.
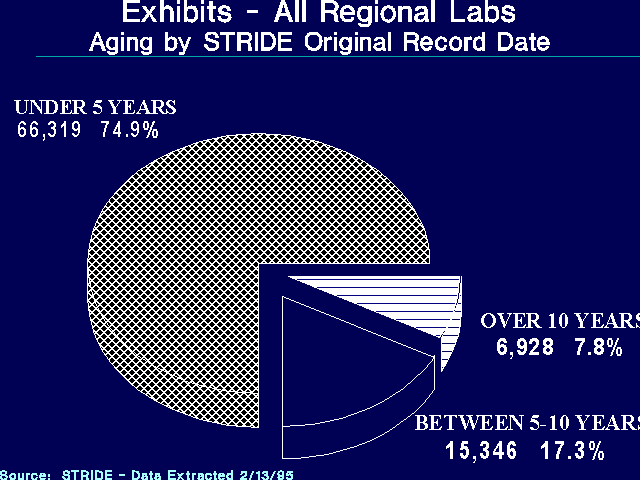
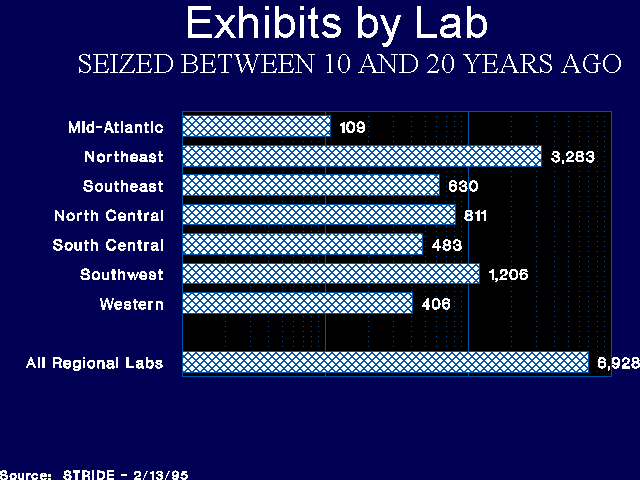
The number of exhibits 10 years old and older varied considerably by laboratory. Only 2.4 percent of the exhibits in the Mid-Atlantic Laboratory were 10 or more years old, while almost 15 percent were 10 years or older in the Northeast Laboratory. The number of drug exhibits seized 10 or more years ago, and 20 or more years ago, by laboratory, are reflected in Figures 4 and 5.

Older drug exhibits remain in DEA evidence vaults for several reasons.
-- In a few cases, lengthy judicial processes, including appeals and civil litigation, may still require retaining these older exhibits for an additional period of time.
-- Some of these exhibits are related to completed cases, i.e., cases in which all judicial proceedings have come to an end, but for which the appropriate paperwork was not processed to request destruction of drug exhibits.
-- Most of these exhibits are related to cases in which one or more subject continues to be a fugitive
Some Drug Exhibits Have Been in DEA Vaults Without Activity for Many Years
Our analysis of STRIDE data shows that DEA does destroy many older drug exhibits, i.e., exhibits over 10 years old. Of more than 125,000 exhibits destroyed by DEA laboratories over the past 5 years, DEA destroyed over 8,000 exhibits (almost 7 percent of exhibits destroyed) that hzad been seized more than 10 years before destruction. Table 3 depicts analysis of exhibits destroyed at each regional laboratory over the past 5 years.
| Exhibits Destroyed by Each
Laboratory Over the Past 5 Years |
|||
| Laboratory | Exhibits Destroyed | Exhibits Over 10 Years Old |
Percent of Total |
| Mid-Atlantic | 8,418 | 1,075 | 12.7% |
| Northeast | 21,767 | 2,538 | 11.7% |
| Southeast | 14,786 | 671 | 4.5% |
| North Central | 14,661 | 1,137 | 7.8% |
| South Central | 21,657 | 592 | 2.7% |
| Southwest | 25,783 | 1,220 | 4.7% |
| Western | 18,660 | 1,146 | 6.2% |
| TOTALS | 125,732 | 8,379 | 6.7% |
Source: STRIDE - Data Extracted 2/13/95
However, a significant number of older drug exhibits have had no activity for many years, and will likely remain in DEA vaults indefinitely unless DEA works with United States Attorneys' Offices to close these cases. The number and proportion of exhibits that have had no activity for over 10 years, by laboratory, can be found in Table 4.
| DEA Drug
Evidence Exhibits With No Activity for 10 + Years As of February 1995 |
|||
| Laboratory | Exhibits With No Activity 10+ Years |
Percent of All Open Exhibits |
Number of Related Cases |
| Mid-Atlantic | 109 | 2.4% | 28 |
| Northeast | 2,986 | 14.5% | 456 |
| Southeast | 538 | 4.49% | 152 |
| North Central | 551 | 4.26% | 92 |
| South Central | 397 | 3.32% | 72 |
| Southwest | 746 | 6.0% | 97 |
| Western | 231 | 1.63% | 39 |
| Totals | 5,558 | 6.27% | 936 |
Source: STRIDE - Data Extracted 2/13/95
The DEA Agent's Manual requires enforcement personnel to meet regularly with United States Attorney's Office personnel to discuss the status of pending cases. DEA agents do not typically use such meetings with United States Attorney's Office officials to discuss whether older drug cases are still viable, or whether drug exhibits in these cases should be maintained. Most United States Attorney's Office officials we spoke with emphasized that decisions to destroy drug evidence exhibits should only be made based upon a case-by-case review. United States Attorney's Office personnel stated that to make informed decisions, they need DEA enforcement offices to provide them with specific case file information including: the identity of any remaining fugitives (and the likelihood of their apprehension), the total quantities of drugs involved, and the availability of witnesses.
Case-by-case reviews of old drug cases during the annual inventory process should establish whether each case is viable, worth prosecuting from the perspective of the United States Attorney's Office, and likely to require drug evidence to prosecute. Based upon our analysis, this would require the review of case information for a relatively small number of older cases -- at present, a total of less than 1,100 cases nationwide. Overcrowding in some laboratory vaults, especially the Northeast and Southwest laboratories, could be significantly alleviated through the review and closure of older cases.
Exhibits Retained for Completed Cases and Non-Drug Evidence Take Up Space
Our analysis of STRIDE and case status system data identified 8,391 exhibits in DEA drug evidence vaults that were for cases listed as closed, or for cases missing case status information. DEA personnel should have reviewed pertinent case information during annual inventories, and determined that many of these 8,391 exhibits were from completed cases. Based upon our review of case file information, we determined that DEA can eliminate at least 2 1/2 percent of all exhibits by removing those remaining in the drug evidence vaults for cases already completed. [ Our analysis of case file information revealed that about 50 percent of the exhibits in DEA vaults for cases coded as "normally closed" in the case status system should be removed from the vaults because these cases have been concluded. About 10 percent of the exhibits for cases coded as "administratively closed," and about 30 percent of exhibits for which there is no related case status information, can be removed because the related cases have been concluded.] This would require DEA personnel to review about 1,800 case files nationwide.
Our analysis of STRIDE data also revealed that 4.6 percent of all exhibits in DEA's drug evidence vaults are "non-drug" exhibits, or exhibits in which drugs were expected but not detected in analysis. To free up space in the drug evidence vaults, we believe DEA should consider removing all exhibits that do not contain drug evidence. These exhibits could be photographed and destroyed, or if needed, stored with other non-drug exhibits for the case.
RECOMMENDATIONS
The Inspections Division recommends that the DEA Administrator:
1. Direct DEA enforcement office officials to work with United States Attorneys' Offices staff to assess the viability of all old drug cases, and promptly prepare all necessary paperwork to expedite the destruction of unneeded drug exhibits.
2. Direct DEA enforcement offices to review the status of all cases for which there are exhibits in the vaults and which have a closed or missing case status code in DEA's case status system. Destruction requests for exhibits from cases determined to be concluded should be prepared promptly and these cases should be formally closed.
3. Implement a policy for the relocation of exhibits from the drug evidence vaults that do not contain drug evidence.
DEA SHOULD BETTER CONTROL DRUG EVIDENCE EXHIBITS SIGNED OUT TO COURT FOR EXTENDED PERIODS
During the course of our field work, we identified exhibits at each laboratory that have been signed out to enforcement agents for extended periods. DEA officials told us many of these drug exhibits are probably not in the possession of the agent who signed for them. These officials suggested that exhibits are sometimes transferred to state and local jurisdictions for non-federal prosecutions and that laboratory records had apparently not been updated for some transfers. To improve accountability for these exhibits, laboratory records should be updated to reflect all such transfers. Exhibits signed out to DEA agents for extended periods that are not in the possession of other law enforcement agencies, or of state and local courts, should be promptly returned to DEA vaults for storage.
Our analysis of STRIDE data identified exhibits at each laboratory signed out to enforcement agents for extended periods. Nationwide, 16 percent of all exhibits signed out to DEA agents have been signed out for 6 to 12 months; 6 percent for 12 to 18 months; 2 percent for 18 to 24 months; and 23 percent have been signed out for more than 24 months. Almost 600 exhibits have been signed out for more than 2 years nationwide (see Figure 6).

The total number of exhibits signed out to enforcement agents, and the number and percentage signed out for more than 2 years, at each laboratory, can be found in Table 5.
We found no evidence that existing DEA procedures provide for periodic confirmation that exhibits signed out to agents are still in the agent's possession. We reviewed case file information for a handful of exhibits signed out for extended periods. In each instance, we found that case files contained documentation of transfer to another law enforcement agency, but that laboratory officials were not properly notified.
| LABORATORY LOCATION |
NUMBER OF EXHIBITS SIGNED OUT | NUMBER OF EXHIBITS SIGNED OUT 2 YEARS OR MORE | PERCENT OF EXHIBITS SIGNED OUT 2 YEARS OR MORE |
| Mid-Atlantic | 157 | 10 | 6.4% |
| Northeast | 477 | 195 | 40.9% |
| Southeast | 201 | 23 | 11.4% |
| North Central | 544 | 63 | 11.6% |
| South Central | 321 | 17 | 5.3% |
| Southwestern | 495 | 203 | 41.0% |
| Western | 287 | 63 | 22.0% |
| Totals | 2,482 | 574 | 23.1% |
Source: STRIDE - Data Extracted 2/13/95
RECOMMENDATION
The Inspections Division recommends that the DEA Administrator:
4. Ensure that exhibits signed out by DEA enforcement agents for extended periods are accounted for and that laboratory records are updated for all exhibits permanently transferred.
DEA SHOULD AUTOMATE GENERATION OF DISPOSITION OF DRUG EVIDENCE FORMS TO IMPROVE EFFICIENCY AND REDUCE THE PAPERWORK BURDEN ON DEA OPERATIONS
During our visits to each DEA regional laboratory, we identified a significant opportunity to improve the efficiency of the paperwork process required to destroy drug evidence and reduce the paperwork burden on DEA enforcement operations.
Existing procedures require DEA enforcement personnel to prepare Disposition of Drug Evidence forms (DEA-48s) to authorize laboratories to destroy drug evidence exhibits at the conclusion of a case. In some offices, agents prepare the forms themselves. Secretaries and group assistants in large offices often do the typing. Most of the completed DEA-48s we found in case files were typed manually.
In some offices, enforcement personnel manually examine hard copy case files to identify all drug exhibits for each completed case. In more computer-oriented offices with access to the STRIDE system, staff query STRIDE for a list of exhibits related to each case. Staff in several DEA laboratories reported agents and other enforcement personnel frequently contact them to obtain a list of exhibits related to completed cases, or to find out whether enforcement had already sent DEA-48s for specific exhibits. One laboratory manager told us he had set up a data base on his personal computer to track DEA-48s received so that he could answer such enforcement inquiries more efficiently.
Laboratory personnel also noted that DEA-48s with incorrect exhibit numbers are received from time to time and this paperwork must be corrected. At a minimum, this requires a phone call or two. In some offices, enforcement staff send corrected DEA-48s to the laboratory.
We believe automating the generation of DEA-48s will improve the efficiency of the process, and reduce the paperwork burden on DEA operations. Based upon our testing, we found that STRIDE contains a reliable listing of drug exhibits associated with each case. We discussed the idea of automating generation of DEA-48s with laboratory and enforcement managers and found little resistance to the idea. In several instances, the idea was enthusiastically received.
A standard computer report could easily be developed and implemented to automatically create all necessary DEA-48s for any case number entered into the system. Automatic generation of DEA-48s by enforcement personnel would not require any changes to existing management controls. An additional record type could be created that would indicate a DEA-48 had been generated for an exhibit, the date on which this occurred, and the identity of the individual generating the form.
The Inspections Division recommends that the DEA Administrator:
5. Strongly consider making the necessary changes to the STRIDE system to generate DEA Form 48s automatically and provide enforcement personnel with limited access to STRIDE to generate DEA-48s.
#####