Innovation Market Analysis After Genzyme-Novazyme
N E R A
Economic Consulting
Federal Trade Commission—Department of Justice Workshop on Merger Enforcement Non-Price Competition and Innovation
Innovation Market Analysis After Genzyme-Novazyme
| Richard T. Rapp, NERA | February 18, 2004 |

What is the Objection to Innovation Market Enforcement?
- Policy premise: Whether the social gain from stopping an R&D merger exceeds the social gain from allowing it is predictable.
- Basis: Analogy to the relationship between competition, quantity and price in goods markets. Consumers almost always gain from competition in goods markets.
- Fallacy: The analogy is false. Reducing concentration doesn’t necessarily increase R&D spending and increasing R&D spending doesn’t necessarily increase the speed and volume of innovation. The prediction is a guess.
N E R A
Economic Consulting
How Markets Work SM
Which of These Statements Makes the Most Sense?
- We limit the use of innovation market analysis to cases involving very small numbers of innovation-competitors in order to be conservative.
- We limit the use of innovation market analysis to cases involving very small numbers of innovation-competitors in order to be radical.
What’s Central to Chairman Muris’Public Statement in Genzyme-Novazyme?
- Policy change: First to monopoly, not market power in future goods.
- With no race to market, the merger would have no impact on pace or order of entry. No delay means that the merger has no negative impact.
- If the Genzyme program fails, Genzyme will pursue the Novazyme program and patients will benefit from the merger of resources.
- If the Genzyme program succeeds, the merged firm doesn’t have the incentive to slow the Novazyme program in order to avoid cannibalization.
- Genzyme may use Novazyme’s R&D to bring other products to market
- Absent the merger, Genzyme would have orphan drug exclusivity and the Novazyme drug would more likely be delayed.
- Commitments to avoid delay are in the structure of the deal:
- Crowley as president is a commitment to not delay.
- Milestone payments and Novazyme insiders provide an incentive to avoid delay.
What’s Central to Commissioner Thompson’s Dissent?
- Calls it “a merger to monopoly” and speaks of “market power over Pompe ERT innovation” despite the fact that market power is not the issue.
- Presumption of anticompetitive effect without theoretical or empirical basis, including misapplication of Horizontal Merger Guidelines.
- Imagines a difference in resource allocation arising from the difference between (1) trying to get the first approval with orphan drug exclusivity and (2) trying to get first approval with orphan drug exclusivity for a longer period for the sake of first-mover advantage.
What’s Central to Commissioner Thompson’s Dissent?
(Continued...)
- Imputes anticompetitive meaning to the act of predicting the delay of the Novazyme product launch by the already merged firm.
- Cites the acquisition price, “a payment of considerable magnitude,” as a reason to suspect innovation suppression without reference to the acquired assets (other than the suppression option).
- Imagines a foregone opportunity because “Novazyme’s research path could conceivably have brought a superior product to this Orphan Drug Act market if it were placed in the hands of a biotech industry member of than Genzyme’s.”
What Is the Future Impact of Current Innovation Market Policy?
- The impact of the Commission’s decision to close the Genzyme-Novazyme investigation is the same as not having an innovation market policy—non-interference.
- This and other smart, self-restrained decisions reassure us that the policy does no harm. Is our sense of security false?
Why Mostly Drug Company Mergers?
- R&D projects are large and discrete.
- FDA regulation deters late entry.
- Inventions are usually products, not just attributes.
- Information from merging firms and FDA is plentiful.
- Companies will cave on any innovation market challenge.
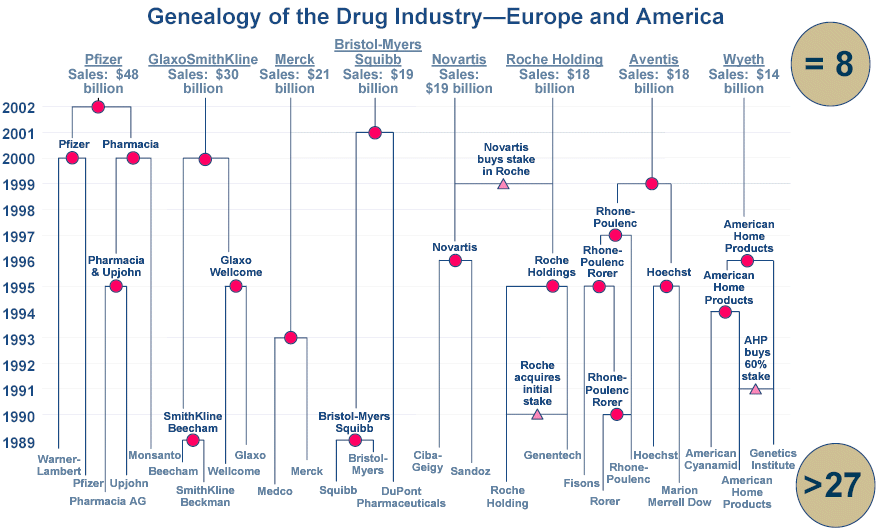
Has Drug Industry Concentration Reduced Innovation?
Parting Shot at Single-Minded Thinking.About Competition and Innovation
On one hand:
On the other hand:
So, an economic historian—in order to tell the story straight—refrains from making an unequivocal judgment about gain and loss from an episode of R&D concentration a century after the fact.
* Tom Nicholas, “Why Schumpeter Was Right: Innovation, Market Power, and CreativeDestruction in 1920s America,” Journal of Economic History, 63/4 Dec. 2003.

 U.S. Department
of Justice
U.S. Department
of Justice